Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
12 augustus 2017: Bron: PLOS one, online september 2014.
Dat immuuntherapie met dendritische celtherapie bij hersentumoren van het type Glioblastoma multiforme (GBM) wel degelijk zin kan hebben blijkt uit een reviewstudie.
De overall overlevingspercentages per jaar geanalyseerd staan hieronder maar op 3 jaar is het verschil 20 % (24% vs 4%) tussen wel of geen immuuntherapie met dendritische celtherapie en op 5 jaar 14% versus 0 procent. Echt een wezenljik en statistisch hoog significant verschil. 1 op de 7 patiënten overleeft een hooggradige hersentumor. Een resultaat dat met geen enkele andere behandeling is te halen bij hooggradige hersentumoren. Nog duidelijker uit deze studies blijkt dat 0 procent een glioblastoma de 5 jaar overleeft. Alle reden dus om ons project Utopie of uitdaging door te zetten.
Hieornder het schema dat de onderzoekers hanteerden in hun studiekeuze. Uiteindelijk bleven er 31 studies over voor de meta-analyse (Tekst loopt verder onder grafiek).
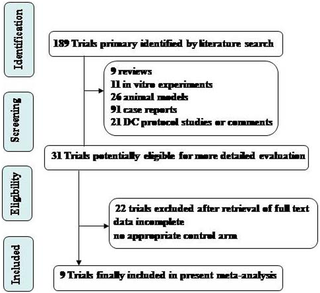
Ik heb niet alle teksten vertaald in het Nederlands maar wel de belangrijkste cijfers vertaald en vet gemaakt in onderstaande teksten:
1 jaar overall overleving (OS):
Information on the 1-year survival was available for seven trials –, –. These seven trials contained 354 patients in total (98 patients received DC therapy, and 256 control patients did not receive DC therapy). De 1 jaar overall overleving (OS) was 82% (80/98) voor glioma patiënten die de DC behandeling hadden gekregen, waar de OS 63% (160/256) bleek voor de patiënten uit de controlegroep. The meta-analysis showed a significantly improved 1-year OS for the patients who received DC therapy compared with those who did not (OR 2.89, 95% CI 1.58–5.27, P = 0.0006). Cochran's Q test yielded a P value of 0.09, and the corresponding I2 quantity was 45% (Figure 2A).
1.5 jaar overall overleving (OS):
Information on the 1.5-year survival was available for six trials –, , . These six trials contained 320 patients in total (80 patients received DC therapy, and 240 patients who did not receive DC therapy served as a control).De 1,5 jaar overall overleving was 59% (47/80) voor glioma patiënten die de DC behandeling hadden gekregen, waar deze 28% (66/240) was voor de controlegroep. The meta-analysis showed a significant benefit for the 1.5-year OS in the HGG patients who received DC therapy compared with non-DC therapy (OR 5.13, 95% CI 2.80–9.41, P<0.00001). Cochran's Q test yielded a P value of 0.35, and the corresponding I2 quantity was 10% (<50%), indicating that the degree of variability between the trials was consistent with what would be expected to occur by chance alone (Figure 2A).
2 jaar overall overleving (OS):
Information on the 2-year survival was available for seven trials –, –. These seven trials contained 354 patients in total (98 patients received DC therapy, and 256 patients who did not receive DC therapy served as a control). De 2 jaar overall overleving was 34% (33/98) voor glioma patiënten die de DC behandeling hadden gekregen en 14% (35/256) voor de controlegroep. The estimated pooled OR for these seven trials showed a significantly increased 2-year OS for the patients who received DC therapy compared with those who did not (OR 4.69, 95% CI 2.48–8.85, P<0.00001). Cochran's Q test had a P value of 0.50, and the corresponding I2 quantity was 0% (Figure 2A).
3 jaar overall overleving (OS):
Information on the 3-year survival was available for six trials –, , . These six trials included 354 patients in total (98 patients received DC therapy, and 256 patients who did not receive DC therapy were used as controls). De 3 jaar OS was 24% (24/98) voor glioma patiënten die de DC behandeling hadden gekregen, versus 4% (10/256) voor de controlegroep. The meta-analysis showed a significantly longer 3-year OS for the patients who received DC therapy compared with those who did not (OR 11.52, 95% CI 4.66–28.45, P<0.00001). Cochran's Q test had a P value of 0.82, and the corresponding I2 quantity was 0% (Figure 2B).
4 jaar overall overleving (OS):
Information on the 4-year survival was available for five trials –, . These five trials contained 320 patients in total (80 patients received DC therapy, and 240 patients who did not receive DC therapy were used as a control). The 4-year OS rates were 20% (16/80) for glioma patients receiving DC treatment and 1% (3/240) for the controls. The meta-analysis showed a significant improvement of the 4-year OS in the HGG patients who received DC therapy compared with those who did not (OR 16.61, 95% CI 5.06–54.52, P<0.00001). Cochran's Q test had a P value of 0.97, and the corresponding I2 quantity was 0% (Figure 2B).
5 jaar overall overleving (OS):
Information on the 5-year survival was available for two trials , . These two trials contained 216 patients in total (42 patients received DC therapy, and 174 control patients did not). De 5 jaar OS was 14% (6/42) voor glioma patiënten die een DC behandeling kregen, versus 0% (0/174) voor de controlegroep. The meta-analysis showed a significantly greater 5-year OS for the patients who received DC therapy compared with those who did not (OR 44.40, 95% CI 5.00–394.16, P = 0.0007). Cochran's Q test had a P value of 0.69, and the corresponding I2 quantity was 0% (Figure 2B), indicating that the degree of variability between the trials was consistent with what would be expected to occur by chance alone.
In onderstaande grafieken zijn bovenstaande resultaten terug te vinden:
The fixed-effects meta-analysis model (Mantel-Haenszel method) was used. OR, odds ratio. DC, DC-containing therapy; non-DC, non-DC-containing therapy. Each trial is represented by a square, the center of which gives the odds ratio for that trial. The size of the square is proportional to the information in that trial. The ends of the horizontal bars denote a 95% CI. The black diamond gives the overall odds ratio for the combined results of all trials.
Het volledige studierapport: Clinical Efficacy of Tumor Antigen-Pulsed DC Treatment for High-Grade Glioma Patients: Evidence from a Meta-Analysis is gratis in te zien.
Hier het abstract van de studie met de referentielijst:
A meta-analysis shows: DC immunotherapy markedly prolongs survival rates and progression-free time, enhances immune function, and improves the efficacy of the treatment of High Grade Glioma patients (GBM)
Clinical Efficacy of Tumor Antigen-Pulsed DC Treatment for High-Grade Glioma Patients: Evidence from a Meta-Analysis

- Published: September 12, 2014
- https://doi.org/10.1371/journal.pone.0107173
Abstract
Background
The effectiveness of immunotherapy for high-grade glioma (HGG) patients remains controversial. To evaluate the therapeutic efficacy of dendritic cells (DCs) alone in the treatment of HGG, we performed a systematic review and meta-analysis in terms of patient survival with relevant published clinical studies.
Materials and methods
A total of 409 patients, including historical cohorts, nonrandomized and randomized controls with HGG, were selected for the meta-analysis.
Results
The treatment of HGG with DCs was associated with a significantly improved one-year survival (OS) (p<0.001) and 1.5-, 2-, 3-, 4-, and 5-year OS (p<0.001) compared with the non-DC group. A meta-analysis of the patient outcome data revealed that DC immunotherapy has a significant influence on progression-free survival (PFS) in HGG patients, who showed significantly improved 1-,1.5-, 2-, 3- and 4-year PFS (p<0.001). The analysis of Karnofsky performance status (KPS) demonstrated no favorable results for DC cell therapy arm (p = 0.23).The percentages of CD3+CD8+ and CD3+CD4+ T cells and CD16+ lymphocyte subset were not significantly increased in the DC group compared with the baseline levels observed before treatment (p>0.05), whereas CD56+ lymphocyte subset were significantly increased after DC treatment (p = 0.0001). Furthermore, the levels of IFN-γ in the peripheral blood of HGG patients, which reflect the immune function of the patients, were significantly increased after DC immunotherapy (p<0.001).
Conclusions
Thus, our meta-analysis showed that DC immunotherapy markedly prolongs survival rates and progression-free time, enhances immune function, and improves the efficacy of the treatment of HGG patients.
Author Contributions
Conceived and designed the experiments: ZXW JXC. Performed the experiments: ZXW JXC XYZ JL-Liu YSL MW DL JL-Li BLX HBW. Analyzed the data: JXC XYZ JL-Liu YSL DL MW JL-Li BLX HBW. Contributed reagents/materials/analysis tools: JXC DL MW. Contributed to the writing of the manuscript: ZXW JXC XYZ JL-Liu.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al.. (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 114(2):97–109. Epub 2007 Jul 6.
- 2. Ruzevick J, Jackson C, Phallen J, Lim M (2012) Clinical trials with immunotherapy for high-grade glioma. Neurosurg Clin N Am. 23(3): 459–470
- 3. Badhiwala J, Decker WK, Berens ME, Bhardwaj RD (2013) Clinical trials in cellular immunotherapy for brain/CNS tumors. Expert Rev Neurother. 13(4): 405–424
- 4. Wilson EH, Weninger W, Hunter CA (2010) Trafficking of immune cells in the central nervous system. J Clin Invest. 120(5): 1368–1379
- 5. Sagar D, Foss C, Baz ER, Pomper MG, Khan ZK, et al. (2012) Mechanisms of dendritic cell trafficking across the blood-brain barrier. J Neuroimmune Pharmacol. 7(1): 74–94
- 6. Xu X, Stockhammer F, Schmitt M (2012) Cellular-based immunotherapies for patients with glioblastoma multiforme. Clin Dev Immunol. 2012: 764213
- 7. Fabry Z, Raine CS, Hart MN (1994) Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol Today. 15(5): 218–224.
- 8. Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 22 12(4): 265–277
- 9. Ardon H, De Vleeschouwer S, Van Calenbergh F, Claes L, Kramm CM, et al. (2010) Adjuvant dendritic cell-based tumour vaccination for children with malignant brain tumours. Pediatr Blood Cancer. 54(4): 519–525
- 10. Ardon H, Van Gool SW, Verschuere T, Maes W, Fieuws S, et al. (2012) Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: results of the HGG-2006 phase I/II trial. Cancer Immunol Immunother. 61(11): 2033–2044
- 11. De Vleeschouwer S, Ardon H, Van Calenbergh F, Sciot R, Wilms G, et al. (2012) Stratification according to HGG-IMMUNO RPA model predicts outcome in a large group of patients with relapsed malignant glioma treated by adjuvant postoperative dendritic cell vaccination. Cancer Immunol Immunother. 61(11): 2105–2112
- 12. Van Gool S, De Vleeschouwer S (2012) Should dendritic cell-based tumor vaccination be incorporated into standard therapy for newly diagnosed glioblastoma patients? Expert Rev Neurother. 12(10): 1173–1176
- 13. Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ (2013) Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev. 39(8): 891–907
- 14. Van Gool S, Maes W, Ardon H, Verschuere T, Van Cauter S, et al. (2009) Dendritic cell therapy of high-grade gliomas. Brain Pathol. 19(4): 694–712
- 15. Mineharu Y, Castro MG, Lowenstein PR, Sakai N, Miyamoto S. (2013) Dendritic Cell-Based Immunotherapy for Glioma: Multiple Regimens and Implications in Clinical Trials. Neurol Med Chir (Tokyo). 53(11):741–754. Epub 2013 Oct 21.
- 16. Wang ZX, Cao JX, Liu ZP, Cui YX, Li CY, et al. (2014) Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 28 20(4): 1095–1106
- 17. Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, et al. (2011) A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 18(8): 1048–1054
- 18. Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, et al. (2005) Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 11(11): 4160–4167.
- 19. Wheeler CJ, Das A, Liu G, Yu JS, Black KL (2004) Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 10(16): 5316–5326.
- 20. Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, et al. (2005) Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 11(15): 5515–5525.
- 21. Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, et al. (2001) Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 50(7): 337–344.
- 22. Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, et al. (2003) Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 89(7): 1172–1179.
- 23. Yu JS, Liu G, Ying H, Yong WH, Black KL, et al. (2004) Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 64(14): 4973–4979.
- 24. Jie X, Hua L, Jiang W, Feng F, Feng G, et al. (2012) Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem Biophys. 62(1): 91–99
- 25. Cho DY, Yang WK, Lee HC, Hsu DM, Lin HL, et al. (2012) Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: a phase II clinical trial. World Neurosurg. 77(5-6): 736–744
- 26. Van Gool S (2013) Immunotherapy for high-grade glioma: how to go beyond Phase I/II clinical trials. Immunotherapy. 5(10): 1043–1046
- 27. Shah AH, Bregy A, Heros DO, Komotar RJ, Goldberg J (2013) Dendritic cell vaccine for recurrent high-grade gliomas in pediatric and adult subjects: clinical trial protocol. Neurosurgery. 73(5): 863–867
- 28. Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, et al. (2008) Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 108(5): 963–971
- 29. Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, et al. (2010) Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 28(31): 4722–4729
- 30. Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 331(6024): 1565–1570
- 31. Wheeler CJ, Black KL, Liu G, Mazer M, Zhang XX, et al. (2008) Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 68(14): 5955–5964
- 32. Hussain SF, Heimberger AB (2005) Immunotherapy for human glioma: innovative approaches and recent results. Expert Rev Anticancer Ther. 5(5): 777–790.
- 33. Ogbomo H, Cinatl J Jr, Mody CH, Forsyth PA (2011) Immunotherapy in gliomas: limitations and potential of natural killer (NK) cell therapy. Trends Mol Med. 17(8): 433–441
- 34. Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell. 140(6): 883–899
- 35. Jackson C, Ruzevick J, Brem H, Lim M (2013) Vaccine strategies for glioblastoma: progress and future directions. Immunotherapy. 5(2): 155–167
- 36. Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, et al. (2013) An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines. 12(6): 597–615
- 37. Li Z, Lee JW, Mukherjee D, Ji J, Jeswani SP, et al. (2012) Immunotherapy targeting glioma stem cells-insights and perspectives. Expert Opin Biol Ther. 12(2): 165–178
- 38. Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, et al. (2011) Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 17(6): 1603–1615
- 39. Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M (2011) Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011: 732413
Plaats een reactie ...
2 Reacties op "Immuuntherapie met dendritische celtherapie voor hersentumoren type glioblastoma multiforme geeft 20 en 14 procent overall overleving versus 4 en 0 procent op 3 en 5 jaar ."
Gerelateerde artikelen
- Gepersonaliseerde vorm van immuuntherapie succesvol bij melanomen gegeven voor operatie doet hersentumor type Glioblastoma Multiforme van arts verdwijnen en er is nog geen teken van recidief na 1 jaar.
- mRNA vaccin doet hersentumoren type Glioblastoma razendsnel veranderen van koude tumoren in warme tumoren. Blijkt uit eerste klinische studie bij volwassen patienten en bevestigt resultaten uit dierstudies
- CAR-T celtherapie rechtstreeks toegediend in de hersenen doet tumorweefsel van Glioblastoma Multiforma snel slinken en soms zelfs verdwijnen.
- DCVax-L verbetert overall overleving en vermindert kans op recidief bij patiënten met nieuw gediagnosticeerd glioblastoom en recidiverend glioblastoom in vergelijking met beste standaardzorg
- Craniopharyngiomas, goedaardige inoperabele primaire hersentumoren van de hypofyse-hypothalamus-as met BRAF V600E mutaties reageren bijzonder goed op combinatiebehandeling van vemurafenib + cobimetinib
- Immuuntherapie met Natural Killer cellen en opgekweekte versterkte autologe T-cellen geeft verrassend goede resultaten bij patienten met een recidief van een hersentumor type glioblastoom multiforme
- Immuuntherapie met cocktail van 9 niet-toxische medicijnen - middelen bij hersentumoren van het type Glioblastoma Multiforme blijkt veilig en geeft veelbelovende resultaten bij eerste 9 patienten uit studie van prof. dr. Halatsch
- Lage tumor mutatie belasting geeft een langere overleving bij patienten met een recidief van een glioblastoom wanneer deze behandeld zijn met immuuntherapie met een gemoduleerd poliovirus of anti-PD medicijn
- Meisje van drie jaar komt en blijft in een complete remissie van een hersentumor glioblastoma met larotrectinib een zogeheten TRK medicijn op basis van haar ETV6–NTRK3 mutatie. copy 1
- Surviving terminal cancer: kunnen overlevenden van een hersentumor helpen kanker te overwinnen? Drie mannen overwonnen hun hersentumor - GBM - en 2 ervan zijn al 15 en 20 jaar vrij van kanker met eigen cocktail van bewezen niet-toxische middelen. copy 1 c
- Immuuntherapie met anti-PD medicijnen (pembrolizumab en / of nivolumab) geeft geen effect op overall overleving bij zwaar voorbehandelde patienten met glioblastoma multiforme
- Immuuntherapie met gemoduleerd poliovirus blijkt succesvol bij recidief van hersentumoren van het type glioblastoma multiforme graad IV
- Inovio Pharmaceuticals Initiates Immuno-Oncology Clinical Study for Glioblastoma in Combination with Regeneron’s PD-1 Inhibitor
- immuuntherapie met rindopepimut een vaccin (gericht op EGFR mutatie) naast temozolomide geeft geen enkel verschil in overall overleving in vergelijking met alleen temozolomide bij hersentumoren Glioblastoma met weinig of geen restweefsel na operatie
- Immuuntherapie met dendritische celtherapie voor hersentumoren type glioblastoma multiforme geeft 20 en 14 procent overall overleving versus 4 en 0 procent op 3 en 5 jaar .
- Dendritische celtherapie bij glioblastoma multiforme graad IV direct na bestraling plus temodal geeft langere overall overleving. 46 procent leeft nog na 3 jaar
- Nivolumab faalt bij recidief van een hersentumor Glioblastoma multiforme. Fase III studie Checkmate 143 wordt stopgezet
- Immuuntherapie met specifiek geprepareerde dendritische cellen (APVACS) plus tetanus virus (CMV) geeft superieure duurzame resultaten op ziektevrije tijd en overall overleving
- Immuuntherapie met gemoduleerd herpesvirus - AdV-Tk therapy - na operatie zorgt voor hoog significant meer en langere overleving in vergelijking met standaardbehandeling bij hersentumoren Glioblastoma multiforme
- APVACS - actief gepersonaliseerde vaccins - ontwikkelen tegen kanker wordt doel van nieuw consortium van 14 organisaties - GAPVAC, waaronder LUMC Leiden
- Toca 511 & Toca FC, een vorm van immuuntherapie met een gemodificeerd virus in combinatie met 5-FU bij hersentumoren - glioblastoma multiforme, heeft de eerste patiënt in behandeling genomen
- Dendritische celtherapie gecombineerd met een vaccin gericht op CD-133 eiwitexpressie voor patiënten met een hersentumor glioblastoma multiforme wordt in fase I/II studie onderzocht
- Behandeling met een virus - Delta-24-RDG - voor hersentumoren - glioblastoma multiforme wordt onderzocht in een fase I /II studie in het VUmc - Amsterdam
- Dendritische celtherapie gecombineerd met zogeheten mRNA's - cellen uit tumoren - verdriedubbeld overlevingstijd van mensen met een hersentumor - glioblastoma multiforme. copy 1
- Vaccin gemaakt van eigen tumorweefsel (HSPPC-96) verlengt leven 2 tot 3 keer zo lang van mensen met een geheel of gedeeltelijke operabele hersentumor. Dit.blijkt uit tussenevaluatie van fase II studie gepresenteerd op ASCO 2011
- Dendritische celtherapie met autoloog vaccin gebaseerd op antigenen geeft significant langere ziektevrije tijd en overall overleving bij patiënten met een hersentumor - glioblastoom
- Dendritische celtherapie vooral ook succesvol toepasbaar bij hersentumoren
- Immuuntherapie bij hersentumoren: Dendritische cellen toegediend na operatie van hersentumoren zorgt voor opmerkelijk goede kansen op geen recidief aldus resultaten uit kleinschalige studie bij 23 patiënten.
- Immuuntherapie bij hersentumoren: Dendritische cellen toegediend na operatie van hersentumoren zorgt voor opmerkelijk goede kansen op geen recidief aldus resultaten uit kleinschalige studie bij 12 patiënten.
- Immuuntherapie met synthetisch vaccin brengt enkele kinderen met een hersentumor - glioblastoom in een totale remissie
- Vaccin dat hersentumoren (Glioblastoom multiforme) met EGFRvIII expressie bestrijdt geeft verdubbeling van overlevingstijd en progressievrije tijd bij nieuw gediagnosteerde patienten
- Neuroblastomen: Immuuntherapie met chimeric anti-GD2 antibody ch 14.18 verhoogt significant overall overleving na 2 jaar met 20% t.o.v. alleen chemotherapie plus stamceltherapie.
- Stamcellen uit eigen bloed naast zware chemokuren geeft kinderen met recidief van hersentumoren langere overlevingstijd en kans op totale genezing, maar ook overlijdt 1 op de 4 kinderen aan de gevolgen van de behandeling
- Virussen: Herpes virusachtig medicijn - G207 wordt in fase I trial onderzocht bij kwaadaardige Glioma tumoren waarvoor radiotherapie - bestraling of chemo niet meer werkt en dus uitbehandeld zijn.
- Immuuntherapie bij hersentumoren met vaccins en virussen waaronder Newcastle Disease Virus. Een overzicht van recente ontwikkelingen.



U kunt ook ons rapport hersentumoren opvragen. Daarin staat alles wat wij weten over recente ontwikkelingen bij hersentumoren: http://kanker-actueel.nl/NL/rapport-hersentumoren-hersenmetastases-actueelnl-actuele-samenvatting-van-recente-ontwikkelingen-bij-hersentumoren-en-uitzaaiingen-in-de-hersenen-van-andere-vormen-van-kanker.html
Met vriendelijke groeten, kees Braam
Webmaster van deze site